Do you have sparse or thin lashes? Do you wish there was a way to grow your own lashes without having to always rely on fake lashes, which can be tricky to apply and don’t always look natural. Are lash extensions too costly to keep up? If you answered yes to any of these questions then Latisse might be a great option for you.
What is Latisse?
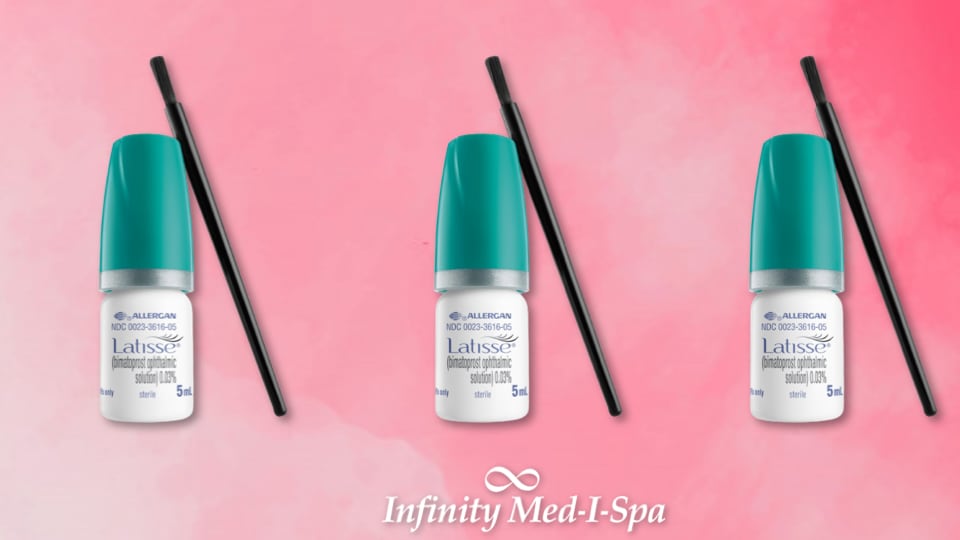
Latisse is an FDA-approved treatment to grow eyelashes for people with inadequate or not enough lashes. It is available by prescription only. It has been studied and proven safe for the treatment of eyelash hypotrichosis. Eyelash hypotrichosis is when an individual has lower than average length, thickness, or number of eyelashes.
Can I Use Latisse If I Wear Contacts?
Absolutely! You simply take your contacts out to apply Latisse. 15 minutes after your application you can put your contacts back in. You don’t have to worry about switching to glasses or not being able to properly see.
How Do I Apply Latisse?
Latisse is applied every evening to your upper lashes only. Before applying your treatment be sure to remove all makeup and other products from the skin and take your contacts out.
Your Latisse treatment will come with special applicators for applying to your lashes. Simply take the applicator and hold it up horizontally. Place a drop of Latisse near the end of the applicator. Then close your eye and brush Latisse onto the base of your upper lashes. Take a tissue and wipe away any excess that got on your skin outside of the lashes. To apply to the other eye, take a fresh applicator and repeat the process. It is important to always use a new applicator for each eye, each day. Applying more often will not improve or speed up your results.
At your visit, you will be given more thorough instructions for how to apply a Latisse treatment. Just know that it’s easy and shouldn’t cause you any problems.
How Long Will It Be Before I See Results?
Optimal results take up to 16 weeks to become evident. You may see results before then but you should use Latisse for 16 weeks to see your full results. Once you begin using Latisse you should continue to use it to achieve your desired results. Once you stop using Latisse, your lashes will go back to their former state.
Who Should Use Latisse?
Latisse is for both men and women who are not happy with the volume or length of their lashes. It should not be used by anyone allergic to any of the ingredients in Latisse. It should not be used as a replacement for mascara. Even though it may darken your lashes it will not act as a mascara substitute.
How Can I Get Latisse?
Latisse can only be prescribed by a licensed physician. Our physicians at Infinity specialize in these types of treatments and would be happy to provide you with care. If you are considering trying Latisse for your lashes, call us today and set up your consultation. Thicker, fuller lashes don’t have to be a dream. They can be your reality.